
Noise induced hearing loss (NIHL) is defined as reduction in auditory acuity (hearing ability) associated with long term exposure to loud sounds. It is the second most common form of sensorineural hearing deficit, after presbycusis (age-related hearing loss). An early term for the condition was “boilermakers’ disease,” because so many workers who made steam boilers developed hearing loss.
There can be two types of hearing loss due to exposure to loud sounds. The first one is temporary threshold shift (TTS). Hearing impairment due to TTS will be a temporary one, from which patient recovers gradually. Depending on duration of exposure, the recovery period may last from minutes to hours or days.
The second type of hearing loss due to noise exposure is a permanent threshold shift (PTS), where elevation in hearing thresholds is a permanent one. This usually occurs following repeated temporary threshold shifts. In some cases, it can happen following a single episode of noise exposure also.
Permanent threshold shift is traditionally divided into two types –
- Acoustic trauma – Situation where a single exposure to an intense sound leads to an immediate hearing loss.
- Noise induced hearing loss (NIHL) –permanent threshold shift because of long term exposure to low intense levels of sounds.
Hearing loss that is caused by the noise exposure due to recreational or nonoccupational activities is termed socioacusis. Hearing loss due to injurious noise at workplace is referred to as occupational noise-induced hearing loss (ONIHL).
Pathophysiology of NIHL
The pathophysiology of noise induced hearing loss is multifactorial and complex. The principle cause of NIHL is damage to cochlear hair cells and associated synaptopathy.
Outer hair cells of the cochlear are more susceptible to noise exposure than inner hair cells. These pathological processes can be broadly divided into ultrastructural mechanisms and metabolic mechanisms.
Ultrastructural mechanisms
The primary pathology behind NIHL is changes to ultra-structural mechanisms inside the cochlea. These changes in micromechanics include depolymerization of actin filaments in stereocilia (in TTS), edema and swelling of stria vascularis, afferent nerve endings and supporting cells inside the cochlea.
Temporary threshold shift is also associated with buckling of supporting pillar cell bodies inside cochlea, while permanent shift is associated with focal hair cell loss and complete degeneration.
Along with the ultrastructural changes, toxic mixing of endolymph and perilymph can also happens in NIHL. When the noise exposure is so severe, a discrete but direct mechanical disruption results in a toxic mixing of endolymph and perilymph through microbreaks in the structural framework of the cochlear duct which leads to loss of hair cells and their corresponding nerve fibers.
A cochlear inflammatory response is initiated in response to acoustic trauma and involves the recruitment of circulating leukocytes to the inner ear.
Apoptotic cell death (programmed cell death) and necrosis has also been observed following noise exposure within 5 -30 minutes following exposure. Death of the sensory cell can lead to progressive Wallerian degeneration and loss of primary auditory nerve fibers.
Metabolic mechanisms
Acoustic overstimulation leads to excessive release of glutamate neurotransmitters in the cochlea. This will lead to depletion of glutathione (GSH), an antioxidant that protects cells from damage due to free radicals.
It is also observed that stimulation of cochlea with sound of moderate intensity increases the cochlear blood flow and sound of high intensity decreases cochlear blood flow. The mechanisms of these phenomenon are unclear, may reflect metabolic demand.
Other less explained, but identified metabolic cochlear mechanisms of NIHL includes outer hair cell plasma membrane fluidity, role of glucocorticoid receptors and oxidative stress.
Predisposing factors for NIHL
Certain people are more prone to the risk of NIHL. Identifying these people and taking necessary actions early can prevent hearing loss in them.
People with variations in form of SNP (single nucleotide polymorphisms) of catalase gene (CAT) and FOXO3 gene, genetic mutations in potassium ion channel genes (KCNQ4 and KCNE1), cadherin 23 (Cdh23) gene, protocadherin 15 (PCDH15), myosin 14 (MYH14), heart shock protein (HSP70) etc are found to have association with NIHL, but further replication in independent sample sets is mandatory.
Other risk factors for noise induced hearing loss include smoking, presence of certain systemic disease like diabetes mellitus, cardiovascular diseases, those who have recreational drug use, chronic lead exposure, those exposed to ototoxic drugs (aminoglycosides, platinum derivatives) and industrial solvents (carbon monoxide, toluene) etc.
When it comes to the characteristics of sound, a sound with frequency of 2-3kHz causes more damage than lower or higher frequencies. Continuous noise is found to be more harmful than exposure to intermittent noise.
Epidemiology
It has been suggested that 12% or more of the global population is at risk for hearing loss from noise, which equates to well over 600 million people. The World Health Organization estimated that one-third of all cases of hearing loss can be attributed to noise exposure.
Researchers have also estimated that as many as 17 percent of teens (ages 12 to 19) have features of their hearing test suggestive of NIHL in one or both ears. In a recent study, it was found that 14.2% of school-aged children in United States showed audiometric characteristics of NIHL.
NIHL usually affects males, while women presents more with acoustic trauma.
No clear-cut differences exist between young and older individuals in their susceptibility to noise-induced hearing loss (NIHL). But the most common age group at clinical presentation is people in early middle age.
Clinical features
Mostly there will be history of long-term exposure to dangerous noise levels may be present. Exposure to more than 85 dB for 8 hours per day is sufficient to the hearing loss.
The most common presenting complaint in NIHL patients is tinnitus, especially in young age group patients and in early course of disease. 40% of these tinnitus sufferers also complaints of hyperacusis. Otalgia, dizziness, headache, sleep disturbance, poor concentration are also other common symptoms. Sometimes they can also present with totally unrelated symptoms like neck pain, shoulder pain and panic attacks.
The hearing loss will be a gradually progressive one over 5-10 years. These people find more difficulty in hearing especially in presence of background noise. Lack of clarity of voice rather than low volume will be their main complaint.
As NIHL progresses, individuals may have difficulty understanding high-pitched voices (eg, women’s, children’s) even in quiet conversational situations. Conversation on the telephone is generally unimpaired because telephones do not use frequencies above 3000 Hz.
Some patients may also have history of social withdrawal, increased reliance on spouse for social and family interaction, depression etc.
Diagnosis
The diagnosis of noise induced hearing loss is usually a delayed one, until the loss became severe enough for patients to volunteer for a hearing exam. At present, there exist no specific test to correctly diagnose NIHL.
The diagnosis is straight forward in an individual with a clear and prolonged history of unprotected exposure to excessive noise, no evidence of any other otologic pathology and audiogram showing classical 4-6KHz dip.
Typically ear examination findings will be normal in these patients.
In 1990, Dobie listed criteria for the diagnosis of occupational noise-induced hearing loss (ONIHL), as follows:
- ONIHL is always a sensoryneural hearing loss which is almost always bilateral.
- High-frequency losses rarely exceed 75 dB, and low-frequency losses rarely exceed 40 dB
- Hearing loss does not progress after noise exposure is discontinued.
- As hearing loss progresses, the rate of hearing loss decreases.
- Loss is always greater at the frequencies 3000-6000 Hz than at 500-2000 Hz. Loss is usually greatest at 4000 Hz. The 4000-Hz notch is often preserved even in advanced stages.
- In stable exposure conditions, losses at 3000, 4000, and 6000 Hz usually reach a maximum level in 10-15 years.
The differentials that needs to be rule out are autoimmune inner ear disease, genetic sensorineural hearing loss, ototoxicity, presbycusis, sudden sensorineural hearing loss, otosclerosis, head injury, meningitis, serious systemic illness, Meniere’s disease etc.
Investigations
Pure Tone Audiometry (PTA)
A pure tone audiometry will show characteristic notching with maximum reduction in sensitivity to stimulation in range of 3-6kHz (4K dip) with some recovery at 8KHz. The origin of this 4-kHz notch in the NIHL audiogram is because of the resonator function of the external auditory ear canal. The notch widens to involve adjacent frequencies at later stages.
Salmivalli et al found that the notch begins at 6 kHz twice as often as it begins at 4 kHz and the 6kHz notch might be the earliest change found in NIHL.
It has to be noted that, the notch may not be characteristic in patient with coexisting hearing loss of other etiology. See below audiogram of noise induced hearing loss.
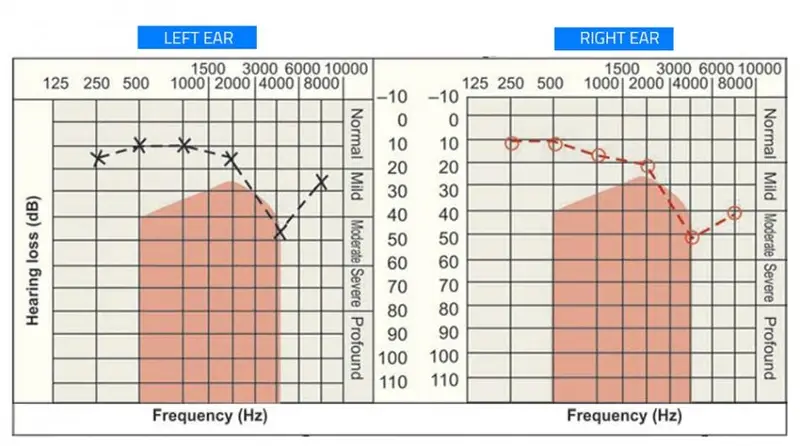
Significant loss at frequencies below 2KHz is extremely uncommon.
Usually the audiogram will be symmetrical for both ears, but unsymmetrical cases can happen due to protective head shadow effect (especially in people like soldiers who uses gun on one particular shoulder).
Speech discrimination score
The speech reception threshold (SRT) should also be measured for each ear. The Speech-recognition scores will be consistent with the audiometric loss. Discrimination score below 60% suggests an etiology other than NIHL.
Tympanometry
The role of tympanometry in NIHL is to confirm normal middle ear function only and to rule out pseudo-deafness.
Otoacoustic Emissions (OAE)
Studies on NIHL with OAE has shown some evidence of decreased contralateral suppression of OAE.
Reductions in OAE levels are more sensitive than pure tone audiometric thresholds in detecting the early states of permanent noise-induced cochlear damage and used as a diagnostic tool for early detection of NIHL.
Brain stem Evoked Response Audiometry (BERA) and Cortical Evoked Response audiometry (CERA)
Role of BERA and CERA in NIHL is limited to individuals whom a significant non-organic component/functional HL is suspected – provides objective measures of hearing thresholds.
MRI of brain with Gadolinium contrast
An MRI is indicated when the hearing loss is assymetrical to rule out presence of any retrocochlear pathologies.
Loudness discomfort level
Measuring the loudness discomfort level is found to be useful in presence of hyperacusis. This also helps the doctor to monitor effects of treatment.
Tinnitus pitch and intensity matching
Has got only little role in assessment of tinnitus severity.
Biomarkers
Prestin and Otolin-1 are inner ear proteins whose blood levels are found to be elevated in NIHL. So far its role has been studied only in preclinical models and human studies are still pending.
Treatment
Once NIHL is diagnosed, further exposure should be avoided. This can be achieved by avoiding the exposure altogether or by use of personal hearing protection methods.
Hearing protectors
- Cotton wool provides 5dB attenuation.
- Ear plugs: When sealed correctly into the ear canal, earplugs reduce the noise that reaches the middle ear by 15 to 30 dB, and they work best for the mid to higher frequency range (i.e., 2 to 5 kHz).
- Earmuffs are more effective protectors, in which noise is attenuated by 30 to 40 dB, especially for frequencies between 500 Hz and 1 kHz. In areas with extremely high noise levels, earplugs do not afford sufficient protection, and individuals should be advised to wear both earplugs and earmuffs.
A poorly fitting hearing protector does not prevent any hearing loss. Hearing protectors should be worn all the time, because if they are removed for even a few minutes, their effective cumulative attenuation capability is severely reduced. Removing hearing protection for only 15 minutes of an 8-hour shift can cut protection efficacy in half.
Active noise reduction
This method uses electronics to provide sound inside a set of ear muffs that is 10 degrees out of phase of ambient sound. This out of phase signals effectively cancels out the background sound.
This is a very effective form of sound attenuation, particularly for lower frequencies and is frequently used in military and aircraft settings. The only limitation is cost factor of the electronics required.
Pharmacological measures
The drug therapy in NIHL is still a topic of active research with limited data on human beings. More about these are drugs are explained under the heading research works in noise induced hearing loss.
Specific management
Binaural hearing aids are recommended in NIHL and they produce approximately additional 10dB sensory average.
Due to the severity of the hearing loss and/or the poor speech recognition due to synaptopathy, some individuals with noise induced hearing loss might eventually become candidates for cochlear implantation (CI) either with full electrical or with electro-acoustic stimulation (EAS).
Nonspecific management
The nonspecific management of noise induced hearing loss includes advices regarding optimization of acoustic environment (e.g.: reduction of background noise, face to face conversation) and an explanation of problem to allow legitimization of hearing loss.
Infrared headphones for TV, volume controllable telephones, Louder doorbells with an alternative alerting system like flash lights or vibrations, hearing dogs, lip reading classes etc are some of the nonspecific management options of NIHL.
Control of underlying comorbid conditions like smoking, cardiovascular disease, diabetes mellitus, hyperlipidemia, exposure to ototoxic drugs are also needed along with annual/ bi-annual audiograms.
Prevention of NIHL
NIHL is the only type of hearing loss which can be completely preventable. Prevention remains the best option for limiting the effects of acoustic trauma.
Regulations, legislation and workplace noise policy
The Occupational Safety and Health Administration (OSHA) mandates that employers provide hearing conservation programs for their employees in workplaces where noise levels equal or exceed 85 dB(A) for an eight-hour time-weighted average. An occupational hearing conservation program includes engineering and administrative controls to reduce noise exposures, employee training in the use of hearing protection and annual audiometry for all workers who are exposed to noise.
Education
- Know which noises can cause damage (those at or above 85 decibels).
- Be alert to hazardous noises in the environment.
- Wear earplugs or other protective devices when involved in a loud activity.
- If you can’t reduce the noise or protect yourself from it, move away from it.
- Protect the ears of children who are too young to protect their own.
- Make family, friends, and colleagues aware of the hazards of noise.
- Have your hearing tested if you think you might have hearing loss.
- Have pre-employment audiometric assessment and annual audiometric monitoring.
Research works
Research works on NIHL are limited to preclinical lab models. All these researches are still in its infancy and much work lies ahead before we can reliably utilize these them for diagnosis and treatment of NIHL. Some of them are listed below
Protection from conditioning the cochlear efferent system – The mammalian cochlea may be capable of actively adapting to certain high-level sounds by undergoing “exposure experience.” The typical conditioning paradigm consists of providing a preexposure training experience using a moderate-level stimulus that, at a more intense level, becomes the subsequent overexposure stimulus.
Pharmacologic protection – Acetyl L Carnitine, N-methyl-D-aspartate, N -acetyl-l-cysteine, ACTH analogues, fluvastatin and D-methionine, are found to be useful for hearing protection in noise exposed animals and prevents further damage of hair cells.
Anti-inflammatory effects of corticosteroids specifically intratympanic dexamethasone, Antioxidant property of drugs like N-acetylcysteine (NAC), ginseng, co-enzyme Q10, as well as several vitamins, such as vitamin A, vitamin C, vitamin E, and vitamin B12 etc are found to be of beneficial in lower animals. The efficacy of these treatments in humans is still unknown.
Src-PTK inhibitors such as KXI-004, KXI-005, and KXI-174 are found to be beneficial in preventing apoptotic cell death when placed on round window membrane of chinchillas.
A recent study by Kayyali et al found that, targeted nanoparticle c-Jun N-terminal kinase (JNK) inhibitor, D-JNKi-1, delivery to OHCs can protect inner ear from noise induced hearing loss (NIHL).
Neurotrophin-3 (NT3) and brain derived neurotrophic factor (BDNF) are important for formation and maintenance of hair cell ribbon synapses in the cochlea, as well as in the vestibular epithelia. Application of NT3 and BDNF on the round window, either directly or as neurotrophin-secreting olfactory stem cells, immediately after noise trauma, can potentially reduce the synaptopathy (indicated by increased number of presynaptic ribbons, postsynaptic glutamate receptors, and co-localized ribbons) and recover hearing.
Dietary protection – Increased levels of plasma concentration of Vitamin C, E and magnesium effectively reduces both PTS and TTS.
Gene therapy – Overexpression of mammalian atonal homolog 1 (Atoh1) leads to an increase in the production of extra-numerary hair cells. Its discovered that new hair cells can be grown in a mature mammalian ear by injecting an adenovirus that carries the Atoh1 gene directly into the endolymph. Most importantly, more recent studies showed that not only did Atoh1 induce repair/regeneration of damaged or lost stereocilia and hair cells in the deafened adult guinea pig, but substantially improved hearing thresholds were also measured.
Similar research by Noda et al from Sunnybrook Research Institute, Toronto, ON, Canada has shown that primary auditory neurons (PANs) can be regenerated from spiral ganglion non-neuronal cells (SGNNCs) by injection of neurogenic pioneer transcription factor Ascl1 and the auditory neuron differentiation factor NeuroD1.
Biomarkers – Role of biomarkers like Prestin and Otolin-1 in noise induced hearing loss has been proposed by Dr. Parham K, Dr. Hana et al etc.
References
- Le TN, Straatman LV, Lea J, Westerberg B. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. Journal of Otolaryngology-Head & Neck Surgery. 2017 Dec;46(1):41.
- McBride DI, Williams S. Audiometric notch as a sign of noise induced hearing loss. Occupational and Environmental Medicine. 2001 Jan 1;58(1):46-51.
- Hana RS, Bawi BL. Prestin, otolin-1 regulation, and human 8-oxoG DNA glycosylase 1 gene polymorphisms in noise-induced hearing loss. Ibnosina Journal of Medicine and Biomedical Sciences. 2018 Mar 1;10(2):60.
- Parham K. Ushering in the era of circulatory otologic biomarkers. Ibnosina Journal of Medicine and Biomedical Sciences. 2018 Mar 1;10(2):37.
- Dobie RA. A method for allocation of hearing handicap. Otolaryngol Head Neck Surg. 1990 Nov. 103(5 (Pt 1)):733-9.
- Verbeek JH, Kateman E, Morata TC, Dreschler WA, Mischke C. Interventions to prevent occupational noise-induced hearing loss: a Cochrane systematic review. International journal of audiology. 2014 Mar 1;53(sup2):S84-96.
- Henderson E, Testa MA, Hartnick C. Prevalence of noise-induced hearing-threshold shifts and hearing loss among US youths. Pediatrics. 2011 Jan 1;127(1):e39-46.
- le Clercq CM, Goedegebure A, Jaddoe VW, Raat H, de Jong RJ, van der Schroeff MP. Association Between Portable Music Player Use and Hearing Loss Among Children of School Age in the Netherlands. JAMA Otolaryngology–Head & Neck Surgery. 2018 Jun 14.
- Kurabi A, Keithley EM, Housley GD, Ryan AF, Wong AC. Cellular mechanisms of noise-induced hearing loss. Hearing research. 2017 Jun 1;349:129-37.
- Agarwal G, Nagpure PS, Pal KS, Kaushal AK, Kumar M. Audiometric notching at 4 kHz: Good screening test for assessment of early onset of occupational hearing loss. Indian Journal of Otology. 2015 Oct 1;21(4):270.
- Blioskas S, Tsalighopoulos M, Psillas G, Markou K. Utility of otoacoustic emissions and olivocochlear reflex in predicting vulnerability to noise-induced inner ear damage. Noise & health. 2018 May;20(94):101.
- Mutlu A, Ocal FC, Erbek S, Ozluoglu L. The protective effect of adrenocorticotropic hormone treatment against noise-induced hearing loss. Auris Nasus Larynx. 2018 May 7.
- Kayyali MN, Wooltorton JR, Ramsey AJ, Lin M, Chao TN, Tsourkas A, O’Malley Jr BW, Li D. A novel nanoparticle delivery system for targeted therapy of noise-induced hearing loss. Journal of Controlled Release. 2018 Jun 10;279:243-50.
- Noda T, Meas SJ, Nogami J, Amemiya Y, Uchi R, Ohkawa Y, Nishimura K, Dabdoub A. Direct Reprogramming of Spiral Ganglion Non-neuronal Cells into Neurons: Toward Ameliorating Sensorineural Hearing Loss by Gene Therapy. Frontiers in cell and developmental biology. 2018 Feb 14;6:16.