Thyroid cancer is the most common endocrine malignancy, and the prevalence of differentiated thyroid cancer is becoming increasing worldwide.
Among the differentiated thyroid cancers (DTC) papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC) are the most frequent subtypes; other rare subtypes are medullary thyroid cancer (MTC) and anaplastic thyroid cancer (ATC).
Though mortality from thyroid cancer is low, the risk of recurrence after thyroidectomy is not insignificant, with studies showing up to 30%.
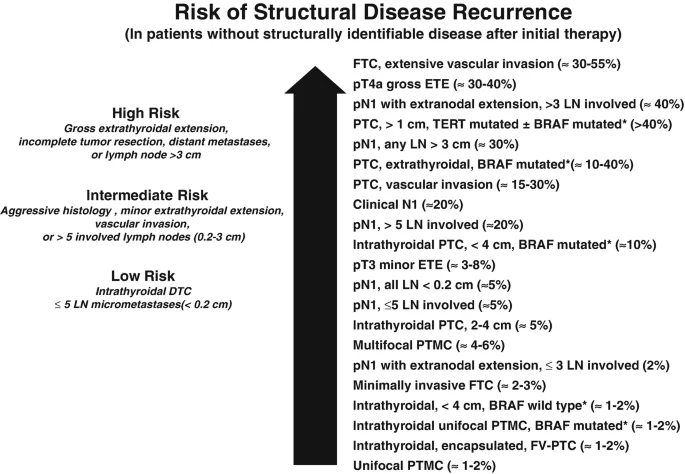
Because the AJCC/TNM staging system of thyroid cancers does not adequately predict the risk of recurrence in differentiated thyroid cancers, the American Thyroid Association (ATA) proposed a three-tiered clinicopathologic risk stratification system which classifies patients into low, intermediate, or high risk of recurrence.
This risk stratification in differentiated thyroid cancers is crucial to avoid overtreatment of low-risk and undertreatment of high-risk patients. It will also help to accurately identify those patients who are at risk of recurrence over the years and tailor their treatment plan accordingly.
Post-Surgery Initial ATA Risk stratification
After the surgery, based on the operative findings and disease extent surgeon stages thyroid cancer. The most commonly followed staging system is the AJCC / TNM staging of thyroid cancers.
Other staging systems for thyroid cancer are the MACIS (Metastases, Age, Completeness of Resection, Invasion, Size) and the GAMES (Grade, Age, Metastases, Extent, Size), AGES, AMES, etc.
To estimate the risk of recurrence, additional clinicopathologic staging systems, such as the American Thyroid Association (ATA) system is followed.
The 2009 American Thyroid Association (ATA) guidelines divided the patients into a three-tiered risk stratification system, based on the risk of disease recurrence.
In the revised 2015 ATA guidelines, the initial ATA low-risk group was extended to include patients with small-volume lymph node metastases (≤5 micro-metastases smaller than 0.2 cm), intrathyroidal encapsulated follicular variants of papillary thyroid cancer, intrathyroidal follicular thyroid cancer with no more than minimal vascular invasion, and intrathyroidal papillary microcarcinomas even if V600E BRAF mutation is present.
In addition to the above, in 2015 ATA high-risk category includes patients with large-volume cervical metastases (≥3 cm) and follicular thyroid cancer with extensive vascular invasion.
2015, ATA guidelines can be summarized as follows:
| Low risk |
|
| Intermediate risk |
|
| High risk |
|
The ATA risk stratification is used for tailoring decisions regarding the need and degree of postoperative thyrotropin [TSH] suppression and the need for adjuvant therapy (like radioactive iodine [RAI] ablation) as well as the frequency and modality of follow-up studies needed. It helps in identifying the risk of structural disease recurrence in patients without structurally identifiable disease after initial therapy.
Dynamic risk stratification
In dynamic risk stratification, the patient’s risk of recurrence is reassessed during every follow-up based on primary therapy, response to therapy and the course of the disease for a minimum period of 2 years.
At each follow-up visit, patients are classified as having one of the following clinical outcomes
- Excellent response – No clinical, biochemical, or structural evidence of disease.
- Biochemical incomplete response – Abnormal Tg or rising Tg antibody values in the absence of localizable disease.
- Structural incomplete response – Persistent or newly identified locoregional or distant metastases.
- Indeterminate response – Nonspecific biochemical or structural findings that cannot be confidently classified as either benign or malignant. This includes patients with stable or declining anti-Tg antibody levels without definitive structural evidence of disease.
The definition of excellent response and biochemical incomplete response is dependent on the primary treatment modality. In patients treated with:
| Initial Therapy | Total thyroidectomy and RAI ablation | Total thyroidectomy without RAI ablation | Lobectomy |
| Excellent response | Non stimulated Tg level <0.2 ng/mL* orstimulated Tg level <1 ng/mL* and undetectable TgAb and negative imaging | Non stimulated Tg level <0.2 ng/mL* or stimulated Tg level <2 ng/mL* and undetectable TgAb and negative imaging | Stable, non stimulated Tg level <30 ng/mL* and undetectable TgAb and negative imaging |
| Biochemical incomplete response | Non stimulated Tg level >1 ng/mL* or stimulated Tg level >10 ng/mL* or increasing TgAb levels and negative imaging | Non stimulated Tg level >5 ng/mL* or stimulated Tg level >10 ng/mL* or increasing Tg values over time with similar TSH levels or increasing TgAb levels and negative imaging | Non stimulated Tg level >30 ng/mL* or increasing Tg level values over time with similar TSH levels or increasing TgAb levels and negative imaging |
| Structural incomplete response | Structural or functional evidence of disease regardless of Tg or TgAb | Structural or functional evidence of disease regardless of Tg or TgAb | Structural or functional evidence of disease regardless of Tg or TgAb |
| Indeterminate response | Nonspecific findings on imaging studies or faint uptake in thyroid bed on RAI scanning or non stimulated Tg level 0.2-1 ng/mL* or stimulated Tg level 1-10 ng/mL* or TgAb levels stable or declining in the absence of structural or functional disease | Nonspecific findings on imaging studies or faint uptake in thyroid bed on RAI scanning or non stimulated Tg level 0.2-5 ng/mL* or stimulated Tg level 2-10 ng/mL* or TgAb levels stable or declining in the absence of structural or functional disease | Nonspecific findings on imaging studies or TgAb levels stable or declining in the absence of structural or functional disease |
| RAI: radioactive iodine; Tg: thyroglobulin; TgAb: thyroglobulin antibody; TSH: thyroid-stimulating hormone. * In the absence of interfering anti-TgAb. | |||
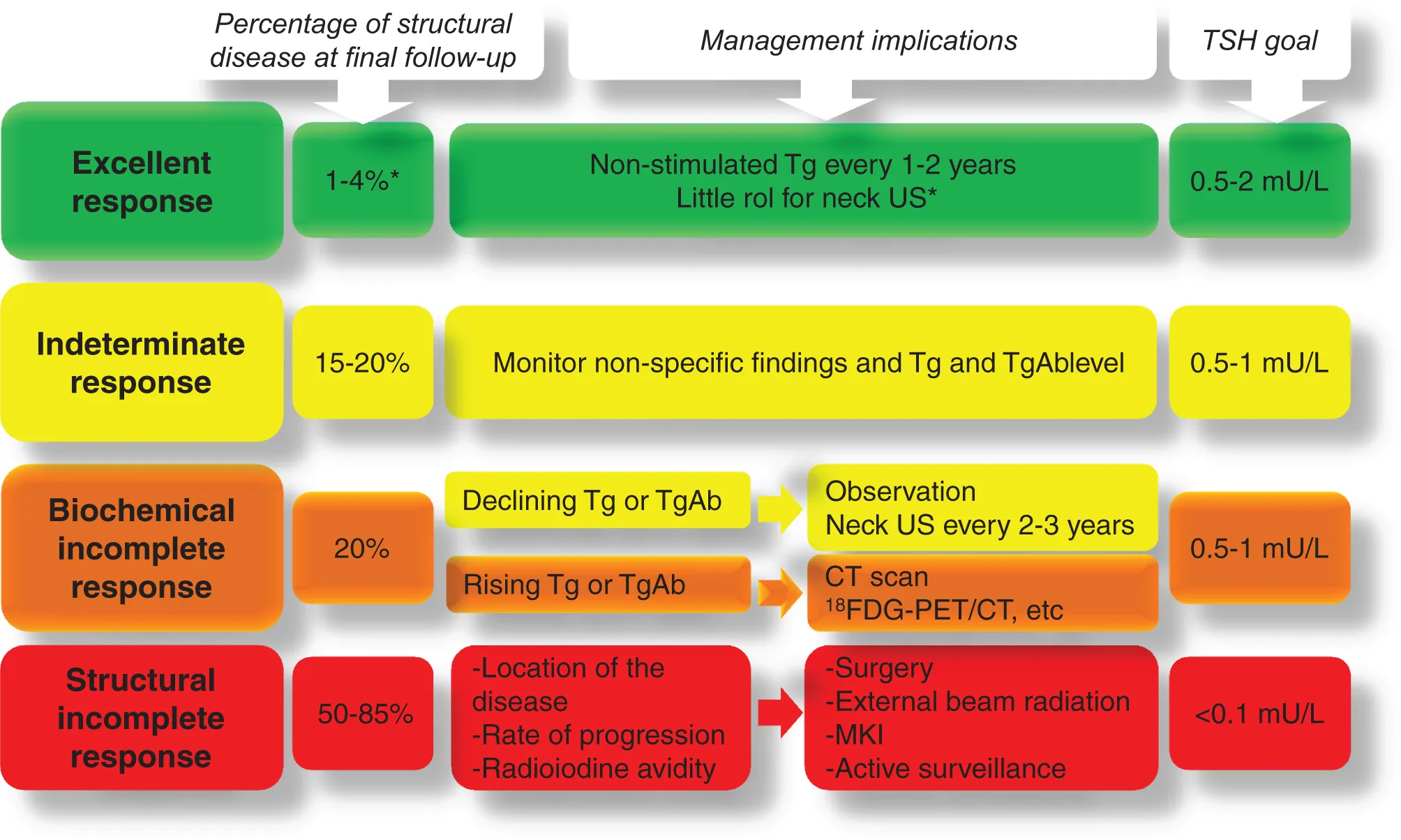
Dynamic risk stratification at each follow-up visit allows the surgeon to tailor ongoing management recommendations to the current clinical status.
According to the guidelines, the following levels of TSH suppression is advised –
- High risk needs to have a TSH level < 0.1mU/L.
- Intermediate risk should have: 0.1-0.5mU/L.
- Low risk who has not undergone remnant ablation and have undetectable serum TSH levels: 0.5-2mU/L.
- Low risk who has undergone remnant ablation and have a low level of serum TSH levels: 0.1-0.5mU/L.
References
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016 Jan 1;26(1):1-33.
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19:1167.
- Tuttle RM, Leboeuf R. Follow up approaches in thyroid cancer: a risk-adapted paradigm. Endocrinol Metab Clin North Am 2008; 37:419.
- Tuttle RM, Tala H, Shah J, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20(12):1341–1349.
- Momesso DP, Tuttle RM. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am 2014; 43:401.