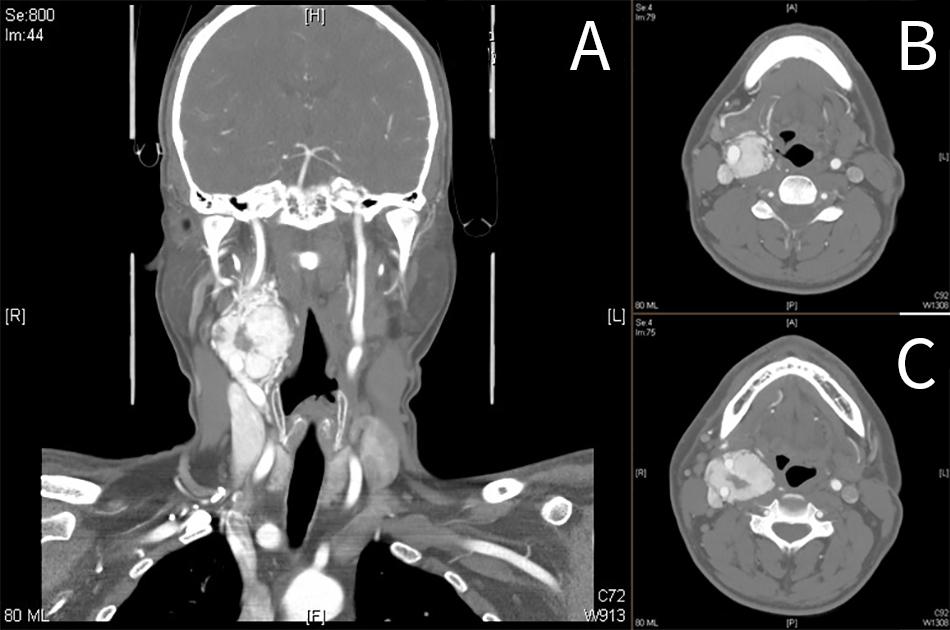
Carotid body tumor, also known as carotid body paraganglioma or chemodectoma, is a highly vascular tumor, arising from chromaffin (paraganglion) cells of the extra-adrenal tissues in the carotid body.
Anatomy
Paraganglions are normally occurring structures found in close association with the sympathetic chain along the aorta and its main branches. Paragangliomas are rare neuroendocrine tumors, arising from neural crests located in extra-adrenal locations, mainly in head and neck region, related to the parasympathetic nervous system. They are found near the arteries and cranial nerves.
The carotid body is a paired, small, reddish-brown, oval structure, located in the posteromedial aspect of the carotid artery bifurcation, first described by von Haller in 1743.
The mean size of the organ is 5 mm × 3 mm × 1.5 mm and the weight ranges between 1 mg to 47.4 mg, with a mean of 12.1 mg in adults.
Its feeding vessels run primarily from the external carotid artery, typically from the ascending pharyngeal artery.
The carotid body is innervated by the Hering’s nerve, a branch of glossopharyngeal nerve originating about 1.5 cm distal to the jugular foramen.
They are highly specialized organs made up of paraganglionic cells which sense pH, pO2, and pCO2 changes in the blood. Thus they are related to autonomic control of the respiratory and cardiovascular systems, as well as blood temperature. Heymans et al. in 1938, received Nobel Prize in Physiology and Medicine for this discovery.
Most literature describes carotid body tumor as located in the adventitia of the carotid bifurcation. But some authors believe that it lies in the peri-adventitial plane of the vessel helping in safe resection of the tumor through the existing space between the vessels and the tumor.
Etiopathology
The exact mechanisms of carotid body tumor formation are still unknown.
Familial cases of carotid body paraganglioma have been shown to be caused by germline mutations of genes associated with the mitochondrial succinate dehydrogenase complex (SDHD, SDHB, SDHC or SDHAF2).
Normal cells generate vascular endothelial growth factor (VEGF), and other growth factors (i.e. erythropoietin) in response to hypoxia. This production is promoted by the stabilization of hypoxia-induced factors (HIF-1 and HIF-2). The disease-induced low partial pressure of the oxygen in the blood, chronic continuous hypoxia of people living in high altitudes (above 1500m), chronic intermittent hypoxia due to sleep apnea syndrome etc. leads to SDH inactivation causing stabilization of HIF, with subsequent downstream activation of VEGF and erythropoietin production, leading to carotid paraganglion hypertrophy.
Types of carotid body tumors
3 different types of carotid body tumors (CBTs) have been described in the literature:
- Sporadic – the most common type, representing approximately 85% of carotid body tumors (CBTs).
- Familial – more common in younger patients (10-15%)
- Hyperplastic – very common in patients with chronic hypoxia, which includes those patients living at a high altitude, those patients with chronic obstructive pulmonary disease (COPD) or cyanotic heart diseases.
Epidemiology
Carotid body tumors are the most common type of head and neck paraganglioma, accounting for 60-70% cases of total cases.
The estimated incidence of carotid body tumors is less than 1 in 30000.
Most commonly carotid body tumors are diagnosed in the 4th to 5th decade. Though the tumor arises in both men and females, like other head and neck paragangliomas the disease has got a higher female predominance (1.9:1).
In about 10% of cases, the tumor is bilateral. In another 7-10% cases, the tumor is familial. Familial tumors are frequently multicentric (35-50%).
Rate of malignant transformation is reported to be 6–12.5% of all cases.
Clinical presentation
Symptoms
The most usual presentation of carotid body tumor is a painless round lateral neck mass, located along the anterior border of upper one-third of the sternocleidomastoid muscle.
These tumors are extremely slow-growing, with a doubling time of 7.13 years and a median growth rate of 0.83 mm/year.
Involvement of cranial nerves will lead to associated symptoms. Most commonly involved cranial nerves are superior laryngeal, vagus and hypoglossal nerves. Hence patient can present with symptoms of cranial nerve palsies like hoarseness, difficulty swallowing, partial paralysis or numbness in the tongue, weakness or pain in the shoulders, vision changes, or a drooping eyelid and bradycardia, etc.
Signs
On palpation, the tumor will be non-tender, there may be transmitted carotid pulsations. As the tumor is encased within the carotid sheath, it can be moved side to side but not up or down (Fontaine sign). Involvement of glossopharyngeal, vagus, accessory, and hypoglossal nerves can lead to specific signs.
A harsh bruit may be heard on auscultation over the tumor.
Sometimes these tumors can secrete catecholamines giving place to hypertensive crises, arrhythmias, palpitations etc.
Other associations of carotid body tumors
Familial carotid body tumors are associated with
- multiple endocrine neoplasias: MEN IIa and MEN IIb
- Carney triad – the coexistence of three types of neoplasms, mainly in young women, including gastric gastrointestinal stromal tumor, pulmonary chondroma, and extra-adrenal paraganglioma.
- phakomatoses
- tuberous sclerosis complex (TS)
- neurofibromatosis type 1 (NF1)
- von Hippel-Lindau disease (vHL)
Differential diagnosis
The most common differential diagnosis are
- cervical lymphadenopathy
- carotid aneurysms
- laryngeal tumors
- metastatic tumors
- cervical lymphadenopathy
- carotid bulb ectasia
- other parapharyngeal tumors like
- vagal schwannoma: tends to displace both vessels together rather than splaying them
- vagal neurofibroma: tends to displace both vessels together rather than splaying them.
- salivary gland tumors
Investigations
Urinary catecholamines
- Usually done in patients having any symptoms suggestive of a functional carotid body tumor.
Doppler ultrasound
- This will allow early and non-invasive accurate detection of tumors with 100% sensitivity even when they are not palpable.
Contrast-Enhanced CT Scan
- soft tissue density on non-contrast CT (similar to muscle)
- bright and rapid (faster than schwannoma) enhancement
- splaying of the ICA and ECA (lyers sign).
Magnetic Resonance Imaging (MRI)
- With T1WI
- lesion may appear iso to hypointense compared to muscle.
- intense enhancement following gadolinium.
- iso to hypointense compared to muscle.
- In T2WI
- classic “salt and pepper” imaging appearance – The “pepper” refers to the low signal flow voids, and the “salt” refers to high signal foci of hemorrhage and/or slow flow on T2.
- hyperintense compared to muscle.
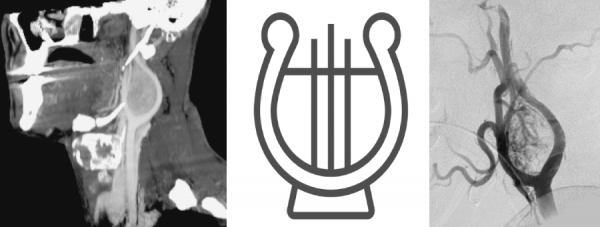
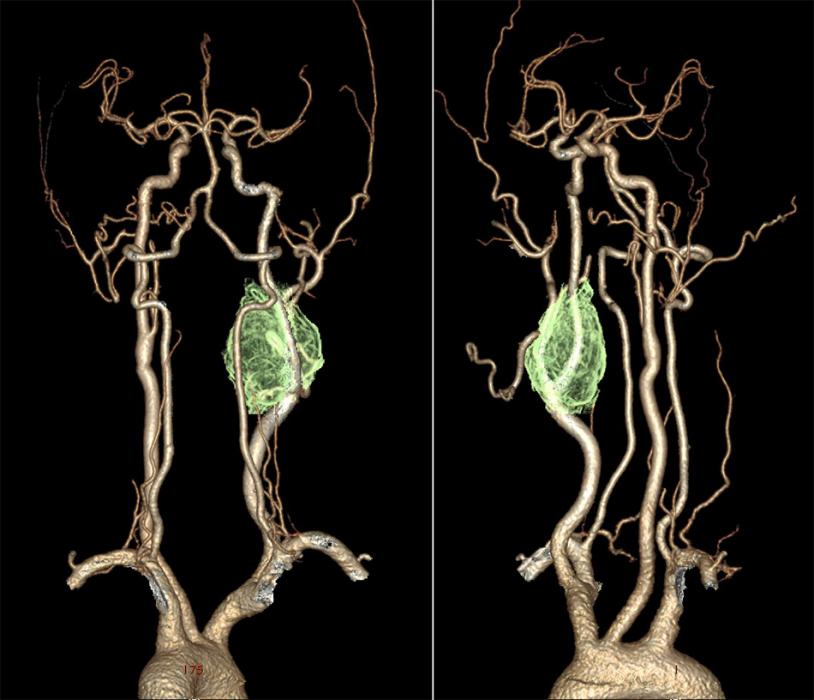
Digital Subtraction Angiography (DSA) – the gold standard in the diagnosis of carotid body tumors.
- In imaging, the lesions splay apart the internal (ICA) and external carotid arteries (ECA). As it enlarges, it will encase, but not narrow the ICA and ECA.
- The splaying of the carotid vessels (lyre sign) is again identified with an intense blush in tumor with and ‘early vein’ seen due to arteriovenous shunting.
- In addition to showing the site, size, and feeding vessels of the tumor, confirming the patency of the Circle of Willis, identifying the presence of other head and neck paragangliomas, DSA can identify the dominant feeding vessel, thereby allowing for preoperative embolization.
Octreotide Scanning
Indium-111 octreotide is a somatostatin analog used for Octreotide scanning, especially for detecting the presence of multicentric or metastatic paragangliomas, and for distinguishing scar from residual tumor after surgery. As these neuroendocrine neoplasms have surface receptors for somatostatin, a focal area of early intense radiotracer uptake will be seen in the region of the paraganglioma.
The investigation is sensitive for detecting tumors greater than 1.5 cm.
PET-CT Scan
Many authors have recently highlighted promising results of 68-gallium-1,4,7,10-tetra-aza-cyclodo-decane-1,4,7,10-tetraacetic acid-1-Nal3-octreotide ((68)Ga-DOTA-NOC) positron emission CT tomography (PET-CT) in detection and characterization of paragangliomas. But larger studies are still needed to verify its performance.
Histopathology
On gross examination, tumors are usually well-circumscribed and may have a pseudo-capsule. The cut surface is typically solid with a smooth, rubbery texture but may display some areas of hemorrhage.
Histologically, paragangliomas have a characteristic appearance often referred to as a “zellballen” growth pattern which refers to a well-developed nested or organoid tumor cells with an intervening stromal component of delicate fibrovascular tissue and supporting cells or “sustentacular” cells at the periphery of the zellballen or cell nests.
These tumor cells (i.e. paraganglioma cells) are predominantly chief cells with round, hyperchromatic nuclei, dispersed chromatin and abundant granular cytoplasm which may range from eosinophilic to basophilic in color.
Immunohistochemistry (IHC)
Occasionally neuromelanin pigment may be seen and amyloid deposition has also been described.
Like adrenal pheochromocytomas, the tumor cells show reactivity with chromogranin and synaptophysin stains by immunohistochemical techniques along with other markers of neuroendocrine differentiation such as CD56 and neuron-specific enolase.
The sustentacular cell population can usually be identified at the periphery of the nests and are thought to be modified Schwann cells; they are spindle-shaped and can be highlighted with S-100 protein staining.
Some troublesome histologic features suggesting malignancy in carotid body tumors are necrosis, extensive capsular or vascular invasion, increased mitotic activity, atypical mitotic figures, loss of a well-differentiated zellballen pattern with loss of the S-100 positive sustentacular cell population and tumor cell spindling.
Screening USG/CT Scans
If patients have a history of neural tumor, family history, or multiple foci of disease, it is recommended to perform preoperative CT scan of the mediastinum and retroperitoneal sonography.
Treatment
The management of head and neck paragangliomas include surgical resection, external beam radiotherapy, and stereotactic radiotherapy.
Radiotherapy:
Radiotherapy is considered to be an alternative treatment modality for patients with high operative risk (if resection of the carotid vessels is anticipated or if a large lesion is fixed or unresectable because of size), who cannot undergo surgery due to extensive involvement, multiple tumors, etc.
Surgery:
Because of unpredictable malignant potential, early and complete surgical resection is often considered as the treatment of choice. Shamblin classification of carotid tumors helps in predicting the prognosis and difficulties for surgical resection.
The first successful excision of carotid body paraganglioma was performed by Albert in 1889. Gordon-Taylor in 1940 described a safe, subadventitial dissection, which is the widest practice nowadays.
Surgical resection
Pre-op preparation
- Patient selection: Shamblin classification of carotid body tumors helps in predicting the prognosis and difficulties for surgical resection.
- Balloon occlusion test of carotids: Usually performed in patients who are at a high risk of carotid resection due to the tumor involvement (Shamblin grade III).
- Grave risk consent: discussion with the patient about the postoperative complications is extremely important; especially risks of IX, X, XI, XII cranial nerve palsies, bleeding, infection, the possibility of a carotid bypass or vein grafts, stroke, and even death.
- Embolization: The role of embolization is still controversial in carotid body tumors. Many surgeons advice preoperative embolization which helps in reducing the blood loss.
An alternative to embolization is the insertion of a covered stent in the external carotid artery.
Surgical procedure
- Resection is usually performed through a longitudinal neck incision along the anterior border of the sternomastoid muscle.
- The internal jugular vein (IJV) is identified and the common facial vein ligated.
- The common carotid artery is dissected to obtain proximal control.
- Cutting into the tumor is sometimes reserved in the advanced Shamblin classes to free the carotid vessels of the encircling tumor and finally securing the feeders. The decision for vascular repair is usually made intra-operatively at this point.
- When resection only is possible, dissection will be done from the side of the tumor less encasing the vessels (whether medial or lateral) and continued till the nearby vessel is freed. This is followed by freeing the carotid bifurcation and progression in a caudo-cranial direction.
- When difficulties are encountered on one side of the tumor, dissection will be continued from the other side. The shift from side to side will be continued to ensure the safest resection of the tumor.
- The hypoglossal nerve, the vagus nerve and sometimes the glossopharyngeal nerve, will be identified away from the tumor and if infiltrated, gradually and meticulously dissected and freed if possible.
Reconstruction
Resection of the carotid body tumor may need some form of carotid resection and reconstruction. Usually, autologous saphenous vein graft, by an end-to-end anastomosis is considered. Other than the saphenous vein, grafts used are femoral artery/vein, cryopreserved arterial homograft, and prosthetic graft using polytetrafluoroethylene (PTFE) or Dacron. Some cases repair of tear can be done by lateral sutures also.
Complications of surgery
Surgical procedure for carotid body tumor excision is a significant challenge because of the frequent complications and difficulties caused by their high vascularity, proximity and possible infiltration of the carotid bifurcation, compression of cranial nerves in the neck, and extension to the skull base. Hence, the resection of tumors by surgeons with experience in vascular reconstruction is recommended.
According to the review published in 2007 by the JVRG (Joint Vascular Research Group), CBT resection is associated with an overall morbidity rate of 33% and a 1% mortality rate.
The most frequent complications associated with carotid body tumor resection surgeries are-
- Nerve injuries are the most common complications followed by
- The superior laryngeal nerve is the most commonly involved one. Damage to this nerve can lead to some degree of aspiration and voice changes (inability to create high-pitched sounds).
- Injury to a hypoglossal nerve can lead to speech and swallowing problems.
- Injury to the vagus nerve results in vocal cord paralysis with resultant hoarseness and increased aspiration risk.
- Postoperative shoulder pain and weakness is typically a result of an accessory nerve injury.
- Other complications of the surgery include cerebrovascular accidents and wound complications.
Prognosis and Malignant transformation
Malignant transformation is encountered in 2-36% of cases. There exists no test that determines benign from malignant tumors. The criterion of malignancy is based on the development of metastases rather than the histologic appearance. Hence, long-term follow up is recommended for all individuals with paraganglioma.
Distant metastases is rare in carotid body tumors. Most common tumor metastasis is to bone, lung, liver, kidneys, pancreas and regional lymph nodes. No metastatic carotid body tumor disease was reported yet.
The estimated 10-year survival rate in malignant paragangliomas is less than 50%.
References
- Torrealba JI, Valdés F, Krämer AH, Mertens R, Bergoeing M, Mariné L. Management of Carotid Bifurcation Tumors: 30-Year Experience. Annals of vascular surgery. 2016 Jul 31;34:200-5.
- Rim Z, Rim B, Houda C, Souheil J, Najeh B, Ghazi B. Paraganglioma of the carotid body: Report of 26 patients and review of the literature. Egyptian Journal of Ear, Nose, Throat and Allied Sciences. 2015 Mar 31;16(1):19-23.
- Gad A, Sayed A, Elwan H, Fouad FM, Eldin HK, Khairy H, Elhindawy K, Taha A, Hefnawy E. Carotid body tumors: a review of 25 years experience in diagnosis and management of 56 tumors. Annals of vascular diseases. 2014;7(3):292-9.
- Lim JY, Kim J, Kim SH, Lee S, Lim YC, Kim JW, Choi EC. Surgical treatment of carotid body paragangliomas: outcomes and complications according to the shamblin classification. Clinical and experimental otorhinolaryngology. 2010 Jun;3(2):91.
- Ma D, Liu M, Yang H, Ma X, Zhang C. Diagnosis and surgical treatment of carotid body tumor: A report of 18 cases. Journal of cardiovascular disease research. 2010 Jul 1;1(3):122-4.
- Wieneke JA, Smith A. Paraganglioma: carotid body tumor. Head and neck pathology. 2009 Dec 1;3(4):303.
- Arya S, Rao V, Juvekar S, Dcruz AK. Carotid body tumors: objective criteria to predict the Shamblin group on MR imaging. American Journal of Neuroradiology. 2008 Aug 1;29(7):1349-54