Chronic rhinosinusitis (CRS) is a clinical condition characterized by inflammation of the paranasal sinuses that typically lasts beyond twelve weeks. It is caused by infections, allergies, and the presence of sinus polyps or a deviated septum.
Signs and symptoms include headache, nasal discharge, swelling in the face, dizziness, and breathing difficulties.
The condition is so common that it affects approximately 12% of the US adult population. The initial treatment options are medical (intranasal corticosteroids, antihistamines, nasal douching, low dose antibiotics, etc.) and functional endoscopic sinus surgery (FESS) is considered when maximal medical therapy fails.
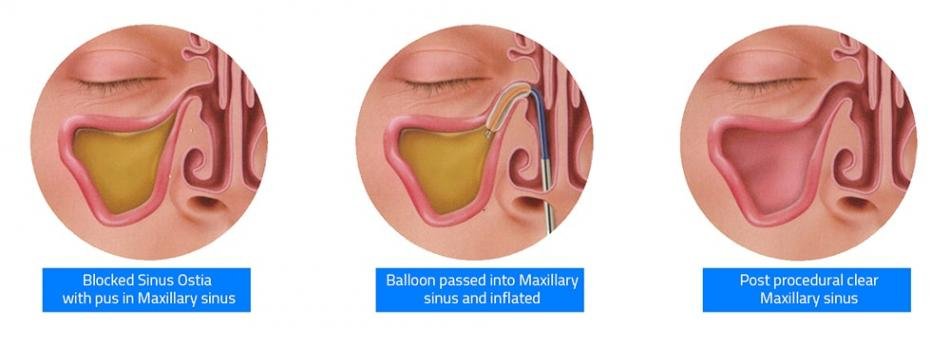
Balloon dilatation of sinus ostia (SOD) for the treatment of chronic rhinosinusitis (CRS) was approved by US-FDA in the year 2015. The inflation of the balloon at high pressure causes compression of sinus mucosa and microfracture of bones surrounding the sinus ostia, improves the sinus drainage and ventilation.
Need for clinical consensus
As there exists no consensus or guidelines for the use of balloon dilation in CRS, wide variation happens in patient selection, and post-procedural management of candidates for SOD, among the otolaryngologists.
For example, anecdotal evidence suggests that some clinicians are practicing balloon dilatation for diseases that range from sinusitis with radiographic abnormalities, to headaches without radiographic abnormalities, to nasal obstruction, to even obstructive sleep apnea.
It was also observed that, during the year 2011-2014, the number of balloon dilatation procedures increased, without a commensurate reduction in traditional functional endoscopic sinus surgery (FESS).
Furthermore, two independent studies, conducted by Gadkaree et al and Eloy et al, found that there is an association between receiving money from the balloon industry and the frequency with which otolaryngologists employ balloon dilatation. Gadkaree et al observed that, of the 294 otolaryngologists performing balloon dilatation procedures, 223 (76%) received payments from a company that manufactures the device.
Payments to otolaryngologists from manufacturers of sinus balloon dilatation devices are associated with the performance of an increased number of such procedures.
This has led to increased scrutiny by payers and providers, which is further intensified by concern over potential inappropriate use of the procedure.
Methods and Results
To address the above issue and to reach in a clinical consensus on the role of balloon dilatation in CRS, an expert panel was constituted by the American Academy of Otolaryngology – Head, and Neck Surgery (AAO-HNS). The panelists included practicing otolaryngologists, members from American Rhinologic Society, American Academy of Otolaryngic Allergy, American Academy of Allergy, Asthma & Immunology, etc.
The target population was adults 18 years or older with chronic or recurrent rhinosinusitis (with or without nasal polyps, with or without prior sinus surgery) for whom SOD is being recommended as a standalone procedure or with endoscopic surgery.
A modified Delphi method was used to distill expert opinion into clinical statements that met a standardized definition of consensus. After 3 iterative Delphi method surveys, 13 statements met the standardized definition of consensus while 45 statements did not. The panel’s statements are listed below.
Clinical consensus statement 2018
- Balloon dilatation should not be done in
- patients who are without sinonasal symptoms or positive findings on computed tomography (CT);
- patients with headache who do not otherwise meet the criteria of CRS or recurrent acute sinusitis
- patients with sleep apnea who do not otherwise meet the criteria of CRS or recurrent acute sinusitis; or
- patients with sinonasal symptoms and a CT that does not show evidence of sinonasal disease.
- There can be a role for balloon dilation in
- patients with persistent sinus disease who have had previous sinus surgery; and
- managing patients with recurrent acute sinusitis as defined in the AAO-HNSF guidelines based on symptoms and CT evidence of ostial occlusions and mucosal thickening.
- A mandatory, pre-procedural CT scanning of the sinuses should be obtained before performing balloon dilation.
- Balloon dilation can:
- improve short-term quality-of-life outcomes in patients with limited CRS without polyposis;
- be performed under any setting as long as proper precautions are taken and appropriate monitoring is performed.
- be performed under local anesthesia with or without sedation; and
- be effective in frontal sinusitis.
- Surgeons who consider reusing devices intended for dilation of the sinuses should understand the regulations set forth by the FDA for reprocessing such devices and ensure that they are followed.
References
- Piccirillo JF, Payne SC, Rosenfeld RM, Baroody FM, Batra PS, DelGaudio JM, Edelstein DR, Lane AP, Luong AU, Manes RP, McCoul ED. Clinical Consensus Statement: Balloon Dilation of the Sinuses. Otolaryngology–Head and Neck Surgery. 2018 Feb;158(2):203-14.
- Eloy JA, Svider PF, Bobian M, Harvey RJ, Gray ST, Baredes S, Folbe AJ. Industry relationships are associated with performing a greater number of sinus balloon dilation procedures. In International forum of allergy & rhinology 2017 Sep 1 (Vol. 7, No. 9, pp. 878-883).
- Gadkaree SK, Rathi VK, Gottschalk E, Feng AL, Phillips KM, Scangas GA, Metson R. The role of industry influence in sinus balloon dilation: Trends over time. The Laryngoscope. 2018 Mar 6.