Cochlear Implant (CI) is a prosthetic electronic device which replaces the function of the damaged inner ear. CI provides direct electrical stimulation to the auditory (hearing) nerve in the inner ear bypassing the damaged hair cells. They are used in patients (both adults and children) with sensory neural hearing loss who are not benefited by conventional hearing aids.
US FDA approved cochlear implants in adults in 1984 and in children in 1990. Since then, around 4,00,000 cochlear implants are estimated to have been performed worldwide. Approximately about 7,000 cochlear implantations take place annually in the United States.
Conventional cochlear implants
The conventional implant consists of an external unit which sits behind the ear and an internal (implanted) unit which is placed surgically under the skin.
The external unit of a cochlear implant consists of a power source, a microphone, a speech processor, and a transmitter. The sound signals picked up by the microphone is analyzed, processed and digitized by the speech processor. The speech processor then passes these signals to a transmitter worn on the head just behind the ear. The transmitter sends these processed signals to the internal unit placed under the skin.
The internal unit includes a receiver and electrodes. The receiver collects the coded electrical signals from the transmitter and delivers them to an array of electrodes which directly stimulates the fibers of the auditory nerve, providing the patient with sound sensation.
Limitations of conventional cochlear implants
The major limitation with a conventional cochlear implant is the psychological and social stigma associated with the external unit.
In addition to the cosmetic aspect, the external unit of a CI is exposed to atmosphere and gravity and is prone to get damaged. The external unit cannot be worn during sleep, and they cannot operate in water (raining, swimming, taking showers) or during some types of physical activities.
Fully implantable Cochlear Implant
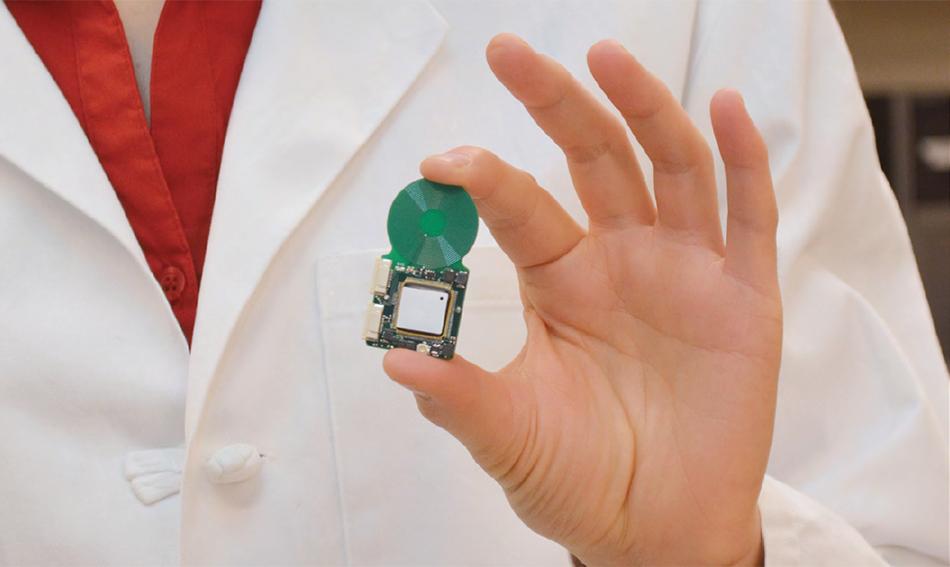
Dr. Konstantina Stankovic, M.D., Ph.D., FACS, an otologic surgeon at Mass. Eye and Ear/Harvard Medical School, and Anantha Chandrakasan, Ph.D. (a renowned expert in ultra-low-power electronics) Head of Electrical Engineering and Computer Science at MIT has recently come up with a prototype of nanotechnology-enabled fully implantable cochlear implant (FICI) which addresses most of the above concerns.
Termed a “System-On-Chip” (SoC), the new cochlear implant prototype integrates various parts of a conventional cochlear implant into a single chip that can be surgically implanted inside the ear.
How fully implantable cochlear implant works?
This fully implantable cochlear implant consists of a piezoelectric sensor front-end (PZFE), a low-voltage reconfigurable sound processor, and an energy-efficient arbitrary waveform neural stimulator and high-voltage electrode switch matrix.
The PZFE is made of the nanomaterial Lead Zirconate Titanate (PZT) and is placed surgically at the umbo of the malleus (a bone inside the middle ear, attached to the eardrum). When sound signals strike the tympanic membrane (eardrum), the umbo is set into back-and-forth vibration which bends the piezo-ceramic material inside the sensor. Bending of the piezo-ceramic material generates a charge across its terminals which is converted to an output voltage by a charge amplifier. This way sound energy is converted to electrical energy.
FICI uses a special sound processor which consumes ultra-low-power for its operation and is highly reconfigurable to enable system power scalability and patient-specific fitting capability. FICI sound processor uses the ubiquitous CIS sound processing strategy.
The energy-efficient arbitrary waveform neural stimulator of FICI receives the processed signals and directly stimulates cochlear nerve fibers and thereby the auditory cortex of the brain, allowing the patient to interpret the original acoustic stimulus as sound. The stimulator requires less power than its peers in use now.
Charging of the chip
The most amazing thing about FICI is that the chip is specially designed to be charged wirelessly through a smartphone while the user is making a phone call or by using a smart pillow. It takes just a few moments to charge the chip completely and the charge lasts for 8 hours.
Advantages of FICI
FICI overcomes all the above limitations of a conventional CI. In addition, as the sensor is not occluding the external auditory canal and it uses the natural sound detecting capabilities of the ear, it can maintain spectral shaping, sound localizing effects of the pinna, the external auditory canal and the tympanic membrane.
Where are we now with the research?
Quoting the words of Dr. Stankovic
“We have a little more work to do to have a fully assembled, packaged device that can be surgically implanted. The next step would be a clinical trial, and we need to show that its performance is at least as successful as the existing devices with the additional advantage of not having external components.”
Though we all are excited about this research, as of today, only a prototype of the system-on-chip paired with sensor and wireless power supply is available. The clinical trial on this and FDA approval is still pending.
Should I wait for FICI to get implanted?
No. You should not wait for FICI as it is still a few years away. Dr. Stankovic said they’d need two to three years to have a packaged device, and then probably around three years more to get FDA approval.
When it comes to cochlear implantation, the earlier the implantation is done the better the results are.
References
- From the Spring 2015 issue of Harvard Otolaryngology magazine.
- Yip M, Jin R, Nakajima HH, Stankovic KM, Chandrakasan AP. A fully-implantable cochlear implant SoC with piezoelectric middle-ear sensor and arbitrary waveform neural stimulation. IEEE journal of solid-state circuits. 2015 Jan;50(1):214-29.