Otitis Media with Effusion (OME), also known as Glue ear, Secretory Otitis Media or Serous Otitis Media is a clinical condition characterized by a collection of non-infected fluid in the middle ear space as a result of a cold, sore throat or upper respiratory infection.
This is a highly common pediatric disease (can affect adults also) that is often wrongly considered to be benign or harmless. However, it has been widely demonstrated that glue ear has both short- and long-term impacts on audition, language/cognitive development, and quality of life.
The most common symptom of glue ear is a temporary hearing loss. It can affect either one ear or both the ears at the same time. Other symptoms may include:
- earache or ear pain
- hearing sounds like ringing or buzzing (tinnitus)
The condition is usually self-limited, which means, the fluid usually resolves on its own within 4 to 6 weeks. But in some children, the fluid may persist for a longer period of time and cause a temporary decrease in hearing, or the fluid may get contaminated or infected with bacteria leading another condition called as acute otitis media, associated with severe ear pain, eardrum (tympanic membrane) perforation and ear discharge.
At present, tympanostomy or ventilation tube (grommet) placement is the recommended treatment for cases of persistent middle ear effusion with an impact on function or on the tympanic membrane. A grommet is a very small tube, which is inserted onto the patient’s eardrum (tympanic membrane) by surgery. This tube helps to drain away fluid in the middle ear. Each procedure usually takes about 30 minutes and is done under general anesthesia to complete.
Currently, the surgical procedure involves a large healthcare team, costly surgical equipment, and set-up in the operating theater, which is an economic burden not only to patients but also to hospitals and healthcare insurers.
CLiKX – A low-cost device for treatment of OME
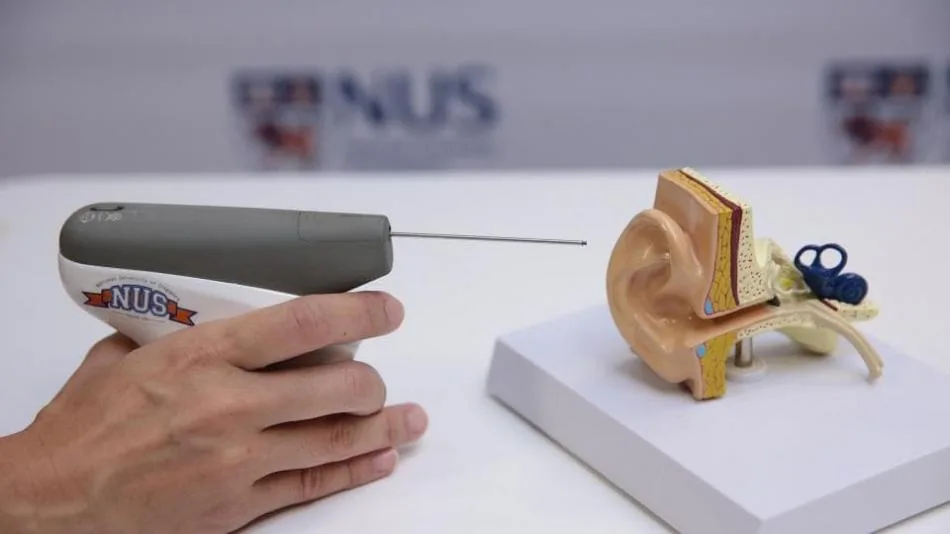
Recently, a team of researchers from the National University of Singapore (NUS) has developed a novel handheld device, known as CLiKX, for the treatment of glue ear.
The interdisciplinary project was started in 2011, and the team was led by Dr. Lynne Lim, an Adjunct Associate Professor from Department of Otolaryngology at the NUS Yong Loo Lin School of Medicine, together with Dr. Tan Kok Kiong, Associate Professor from the Department of Electrical and Computer Engineering at the NUS Faculty of Engineering. It involved five researchers from the engineering and medical facilities of the NUS.
CLiKX is a sensor-guided one, thus making it more precise and easier to use, thereby significantly improving the current surgical treatment of the condition. With a pistol-like applicator, the grommet tube can be easily inserted into a patient’s ear within a single click. In less than a second, the procedure can be done.
Compared to the conventional surgical methods, the advantages of CLiKX are many
- It’s a simple procedure which can be administered in a doctor’s consultation room under local anesthesia, or out of the operating theatre under intravenous sedation without the need for general anesthesia.
- Risks of general anesthesia can be avoided.
- Preoperative preparation and postoperative recovery time for patients are significantly reduced.
- Costs, manpower, and resources can be lowered substantially, and this, in turn, would be welcomed by patients, healthcare institutions, and insurers.
The motivation behind the development of CLiKX is to significantly reduce the recovery time and treatment cost for patients. By streamlining the manpower and resources required for surgical treatment of otitis media, healthcare resources could be deployed more efficiently for other treatments and procedures in hospitals – Dr Tan Kok Kiong.
The palm-sized device weighing about 185 grams is a battery-powered one, which significantly improves current surgical treatment of Otitis Media with Effusion (OME) or ‘glue ear’.
Human trial and commercialization
The project is currently in its early phase only, with promising results. The NUS team aims to conduct the first human trial in Singapore in 2018. They are planning to work with partners to further develop and commercialize the device so that they can launch the device in the market by 2020.
References
- Rosenfeld RM, Shin JJ, Schwartz SR, Coggins R, Gagnon L, Hackell JM, Hoelting D, Hunter LL, Kummer AW, Payne SC, Poe DS. Clinical practice guideline: otitis media with effusion (update). Otolaryngology–Head and Neck Surgery. 2016 Feb;154(1_suppl):S1-41.
- NUS Article on CLiKX